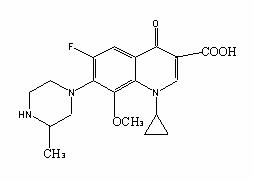
Gatifloxacin
CAS: 112811-59-3
Gatifloxain is [(±)-1-cyclopropyl-6-fluorin-1, 4-dihydro-8-methoxyl-7-(3-methyl-1-piperazine)-4-oxygen -3-guinoline carboxylic acid] 3/2 hydrate.
Trait:Gatifloxain is white or yellow crystallinity powder, odorless, a little bitterness.Not dissolves in diethyl ether, very slightly soluble in water or carbinol or ethanol, slightly soluble in chloroform or acetone, dissolves in 0.1mol/L hydrochloric acid or sodium hydroxide, freely soluble in glacial acetic acid.Melting point: The melting point of Gatifloxain is 181-188℃
Differentiation:(1) To 10mg Gatifloxain, 0.5ml hydrochloric acid and hydroxylamine acid test solution and 2ml 0.4% sodium hydroxide solution are dropped, then adding in 2ml muriate acid, shaking, dropwise ferric chloride test solution, then it becomes salmon pink.
(2) Gatifloxain has the differentiation reaction of organofluoride.
(3) To appropriate amount of Gatifloxain, 0.1mol/L hydrochloric acid solution added in order to make 6 µg/ml solution, assaying it with the spectrophotometry, at the 292nm of wavelength absorbing maximum, at the 263nm of the wavelength absorbing minimum.
(4) The infrared light absorb atlas of Gatifloxain is accordance with that of reference substance.
Censorship:
Clarity of solution:To 50mg Gatifloxain, 10ml 0.4% sodium hydroxide solution are dropped, the solution should be lucidification, if not, comparing it with NO2 turbidity standard color solution, it should be thinner than latter.
Color of solution:Sodium hydroxide solution (1mol/L) dropping into Gatifloxain to make 50mg/ml solution, if it has chromogenic reaction, comparing it with Kelly or yellow NO6 standard color solution, it should be thinner
Acid-base scale: the PH is 7.0~8.0.
Fluorin: the content of fluorin is 4.5%~5.5%.
Related materials: 0.041%.
Moisture: the result is 6.0-8.0%.
The burning residue: the residue should be less than 0.1%.
The heavy metal: the content of heavy metal fewer than 20 millionth.
Content test: 98.5~101%.
Category: Antimicrobial drug
Store:To obscure, airproof, save in a dry place
Preparation:Gatifloxain tablet, capsule, injection
Validity:Interim 18 months